

- [email protected]
+91 40 2344 5962 - View Stock Quote


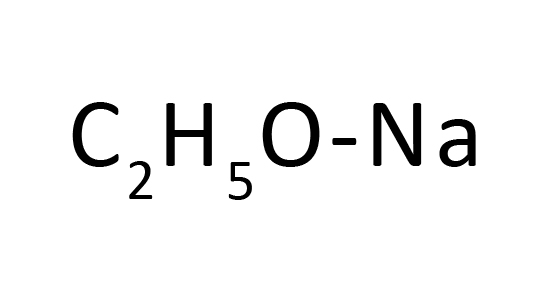

Synonyms : Sodium Deuteride
CAS No. : [7646-69-7]
Molecular Formula : NaH
Formula weight : 24
Sodium hydride is the chemical compound with the empirical formula NaH. This alkali metal hydride is primarily used as a strong, yet combustible base in organic synthesis. NaH is a base of wide scope and utility in organic chemistry. As a superbase, it is capable of deprotonating a range of even weak Bronsted acids to give the corresponding sodium derivatives. Typical "easy" substrates contain O-H, N-H, S-H bonds, including alcohols, phenols, pyrazoles, and thiols.
Sodium Hydride is basically an alkali metal hydride with the empirical formula of NaH. This chemical compound is considered a strong base which is widely used in organic chemistry. Sodium hydride is also considered as saline hydrides or salt-like hydride because it is composed of Na+ and H- ions unlike the other molecular hydrides such as methane, ammonia, etc. It is highly insoluble in organic solvents and owing to this property all reactions involving NaH occur at the surface of the solid.
The preparation of Sodium Hydride involves placing of the cleaned metallic sodium in steel, porcelain tray or a hard- melted glass tube technically called a reactor. Prior to the reaction takes place the air is removed from the reactor by passing hydrogen. The metal tray with sodium is heated to 370o C while the hydrogen used is purified by drying over phosphorus pentoxide and passing it through the melted sodium. The end product of the reaction that is the sodium hydride can be collected as it condenses on the colder parts of the tube in the form of a white plaque.
When Sodium hydride is prepared by such method it contains a small amount of metallic sodium as an impurity. Another important fact about this chemical compound is that the pure crystalline white form of it ignites at 230o C in an atmosphere of oxygen.
Na+H-